WHO revises COVID guidelines: What to know
MANILA, Philippines—Ahead of the meeting set later this month to decide whether the COVID-19 pandemic still presents a global emergency, the World Health Organization (WHO) has updated its COVID guidelines on masks, treatments, and patient care.
Early in the pandemic, from January 10 to 12, 2020, WHO published a comprehensive set of guidelines covering topics related to the management of the outbreak of SARS-CoV-2—the virus that causes COVID-19.
The guidelines included the interim clinical management of the novel coronavirus disease, or COVID-19, which was later updated on June 17, 2020. The then-updated version included new recommendations on the criteria for discharging patients from isolation.
Three years into the pandemic, the UN agency has again revised its COVID-19 guidelines as “part of a continuous process of reviewing such materials, working with guideline development groups composed of independent, international experts who consider the latest available evidence and the changing epidemiology.”
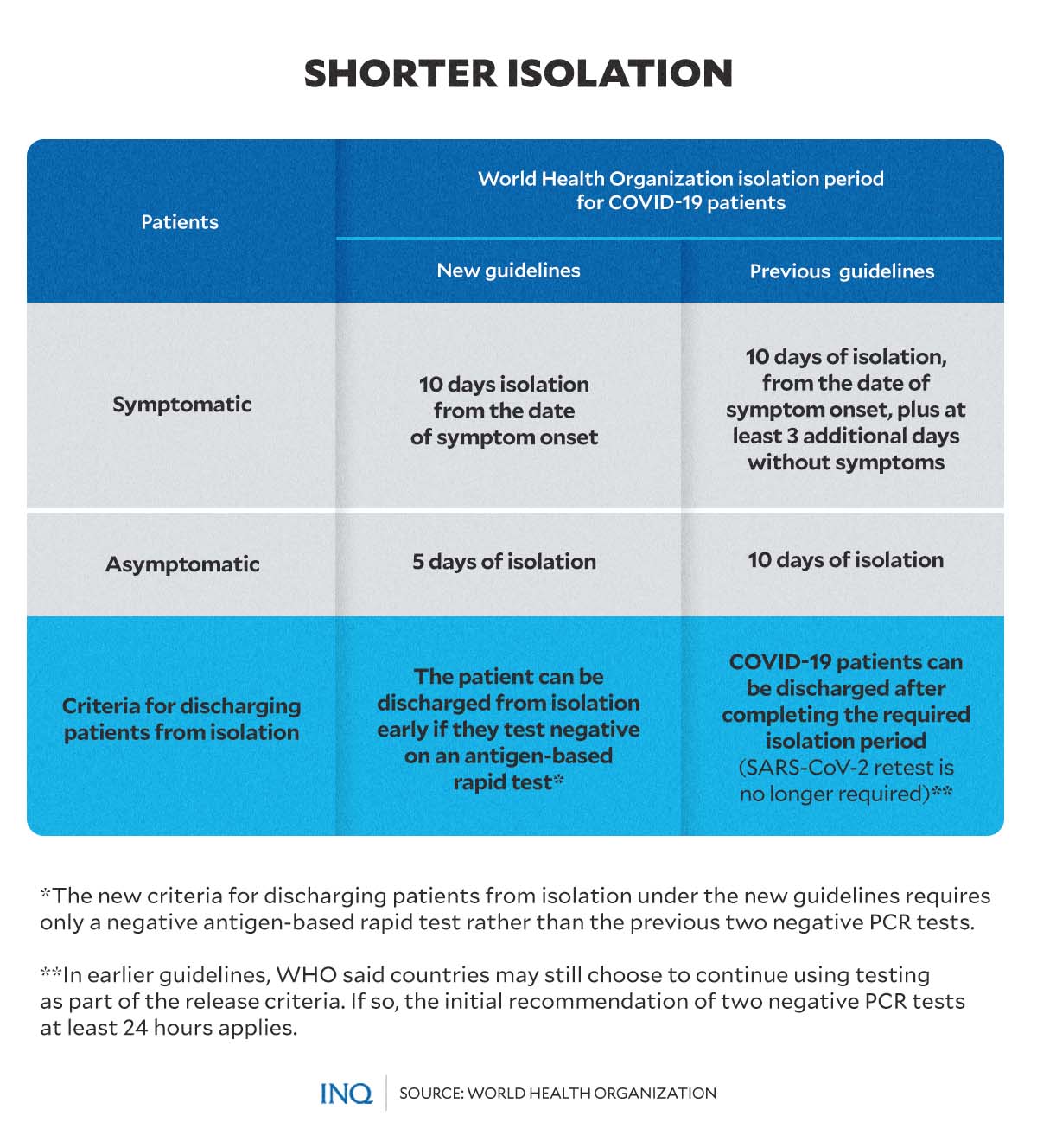
Adjusted isolation period
In the recently published COVID-19 guidelines, WHO adjusted and reduced the recommended isolation period for COVID patients and the criteria for discharging patients from isolation.
“Isolation of people with COVID-19 is an important step in preventing others from being infected. This can be done at home or at a dedicated facility, such as a hospital or clinic,” WHO said.
For symptomatic patients, the new WHO guidelines suggested 10 days of isolation from the date of symptom onset.
In the new guidelines, WHO suggested 5 days of isolation for patients who tested positive for COVID but do not have symptoms.
The previous guidelines advised patients with symptoms to be discharged 10 days after symptom onset, plus at least 3 more days since after the symptoms disappear. For asymptomatic cases, patients can be discharged after completing the required 10 days of isolation.
“The evidence considered by the guideline development group showed that people without symptoms are much less likely to transmit the virus than those with symptoms,” WHO explained.
“Although of very low certainty, evidence also showed that people discharged at day 5 following symptom onset risked infecting three times more people than those discharged at day 10,” it added.
The new guidelines also noted that a patient could be discharged from isolation early if they test negative in an antigen-based rapid test—different from the initial versions of the guidelines, which required negative PCR-type tests.
The June 2020 iteration of WHO’s guidelines required patients—both symptomatic and asymptomatic—to complete the recommended isolation period. The criteria for discharging patients from isolation did not necessarily require retesting.
However, for countries who choose to continue using testing as part of the release criteria, WHO had recommended two negative PCR tests at least 24 hours apart.
Continue wearing masks
In the new guidelines, WHO still recommends the use of face masks as a key tool against COVID-19. WHO reminded the public to continue wearing face masks in specific situations, “irrespective of the local epidemiological situation,” given the current spread of COVID-19 globally.
WHO recommended the use of face masks:
- following recent exposure to COVID-19
- when someone has or suspects they have COVID-19
- when someone is at high risk of severe COVID-19
- for anyone in a crowded, enclosed, or poorly ventilated space.
WHO reiterated its previous statement, explaining that there are other instances when a face mask may be suggested based on a risk assessment. The UN agency listed some factors to consider when deciding to wear face masks, including:
- the local epidemiological trends or rising hospitalization levels
- levels of vaccination coverage and immunity in the community
- the setting people find themselves in.
Last week, WHO officials urged countries to consider recommending passengers on long-haul flights to wear face masks, given the rapid spread of the XBB.1.5 sub-variant—the latest Omicron mutation.
READ: WHO urges travelers to wear masks as new COVID variant spreads
READ: Explainer: What do we know about COVID variant XBB.1.5?
In the Philippines, the government has yet to rescind Executive Order (EO) No. 7—which allows optional face mask use in indoor and outdoor settings—despite recommendations from health experts following the detection of XBB.1.5 in many countries and the feared surge in COVID cases in China.
READ: As world in the dark on China’s COVID nightmare, PH weighs options on arrival
While the use of face masks is optional in indoor and outdoor settings under EO No. 7, the measure stressed that wearing face masks is still mandatory in healthcare facilities, medical transport vehicles, and public transportation.
The elderly, persons with comorbidity, immunocompromised individuals, pregnant women, unvaccinated individuals, and symptomatic persons are also still encouraged to wear face masks.
READ: Expert: Masks a must with new variants
Global emergency or not?
The new guidelines were released ahead of the meeting on January 27, where a WHO committee will determine whether COVID still presents a global emergency—three years after the UN agency declared the disease as a public health emergency of international concern (PHEIC).
In October 2022, WHO’s emergency committee on COVID-19 met and concluded that the pandemic still constitutes a PHEIC. It also noted that it is too early to lift the highest level alert for the COVID-19 crisis.
Last month, at the third anniversary of the original COVID-19 outbreak, WHO expressed high hopes that the disease would no longer be a public health emergency in 2023.
“[W]e have come a long way. We are hopeful that at some point next year, we will be able to say that COVID-19 is no longer a global health emergency,” WHO chief Tedros Adhanom Ghebreyesus said.
READ: WHO eyes end to COVID-19 emergency in 2023
However, Seth Berkley—GAVI global vaccine alliance’s head—suggested that it was still too early to call an end to the COVID-19 emergency.
According to reports, several scientists and WHO advisers also believe it may be too early to declare the end of the COVID-19 pandemic emergency phase—considering the recently reported high levels of infections in China after the country dismantled its zero-COVID policy.
READ: ‘Be prepared, don’t panic’, PH told as China bares COVID numbers
The Philippines’ Department of Health (DOH) said it is prepared for any possibility following the WHO’s emergency committee later this month.
“Even when the public health emergency has been lifted, we know that the virus is here to stay. The Philippines will continue to be cautious and vigilant, and we will still be imposing the same restrictions that we have right now,” said Health Undersecretary Maria Rosario Vergeire, DOH OIC.
READ: ‘Very small probability’ that state of calamity be extended in PH — DOH
Data from the DOH showed that as of January 18, the country’s COVID-19 caseload has reached 4,071,013—with 11,031 tagged as active cases, while 3,995,345 recovered, and 65,637 fatalities.
COVID-19 treatments
Aside from its updated guidelines on COVID-19 isolation and the use of face masks, WHO has also extended strong recommendation for the use of nirmatrelvir-ritonavir—also known by its brand name Paxlovid—for treating COVID-19 patients.
WHO advised pregnant or breastfeeding women with non-severe COVID to consult with doctors to determine whether they are qualified to take Paxlovid.
The drug, according to WHO, had been first recommended in April 2022.
“WHO strongly recommends its use in mild or moderate COVID-19 patients who are at high risk of hospitalization,” WHO said. It added that the first generic producer of the drug had been “prequalified” by WHO.
WHO also kept its recommendation against the use of sotrovimab and casirivimab-imdevimab for treating COVID, citing the medicines’ lack or diminished effectivity against currently circulating variants.
READ: Philippines in talks with Pfizer for procurement of antiviral COVID-19 pill
READ: FDA approves generic version of Pfizer’s COVID-19 pill Paxlovid
RELATED STORIES:
WHO working with China on Lunar New Year COVID-19 risks
Bivalent vax purchase hit ‘roadblock’ as COVID-19 state of calamity in PH not extended
TSB
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link.
Subscribe to INQUIRER PLUS to get access to The Philippine Daily Inquirer & other 70+ titles, share up to 5 gadgets, listen to the news, download as early as 4am & share articles on social media. Call 896 6000.