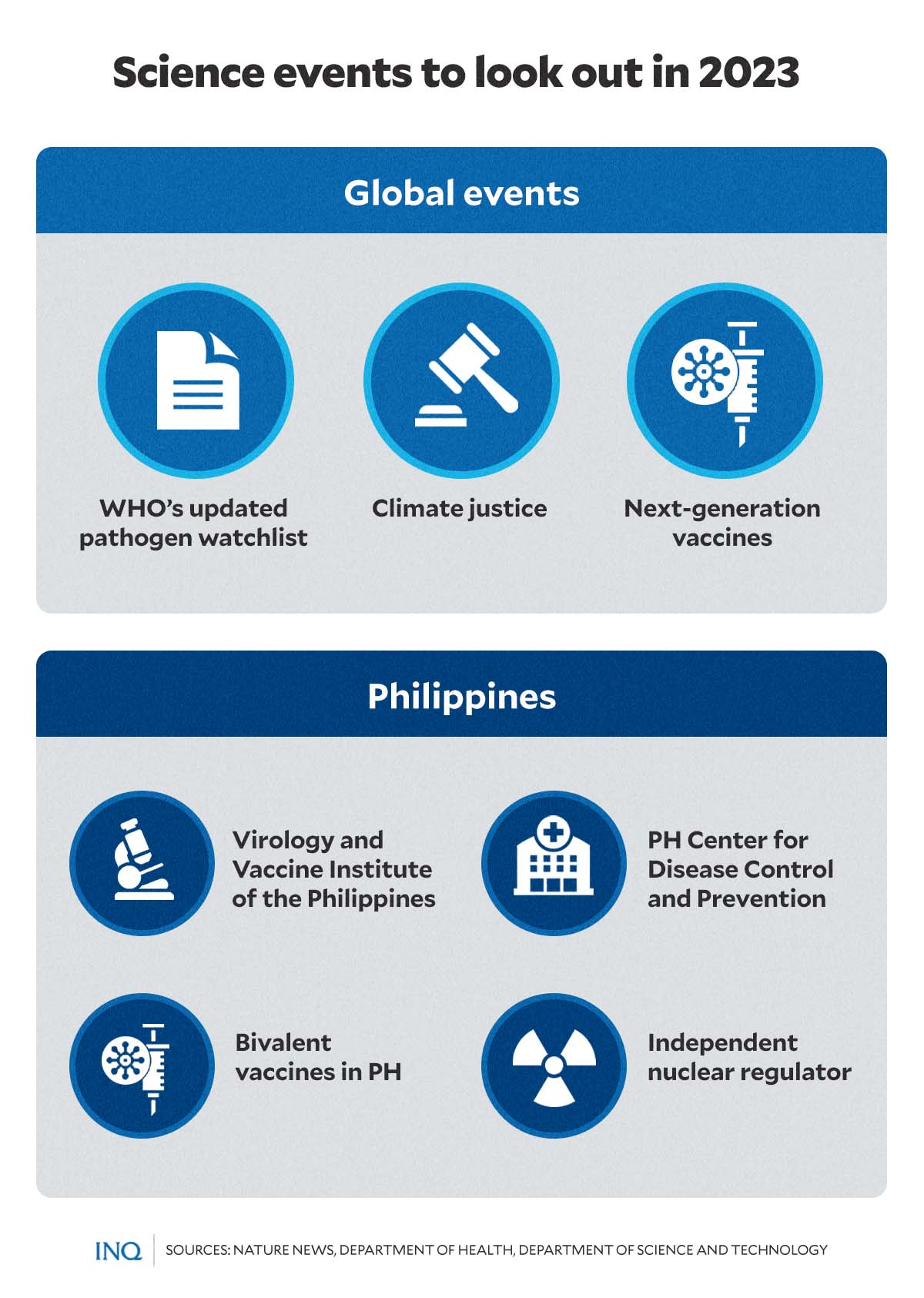
What’s next in science: Look out for these in 2023

FILE PHOTOS
MANILA, Philippines—This year had some of the biggest science events—from the release of new studies on climate change, emerging SARS-COV2 variants and sub-variants, and monkeypox to the creation of bivalent vaccines and significant developments in the study of several other diseases.
Three years into the COVID-19 pandemic progress has been made in terms of understanding how SARS-COV2—the virus that causes the disease—affects the human body, especially in long-term scenarios, which scientists and health experts called “long COVID” cases.
READ: Understanding ‘long COVID’ and how to prevent it
As newer and more transmissive COVID virus variants and sub-variants emerged, huge pharmaceutical companies tested and began rolling out a new tool against it: updated booster shots—also known as “bivalent vaccines.”
READ: What to know about ‘bivalent vaccines’ that target Omicron
In the Philippines, aside from pandemic-related studies, there has also been a focus on science reports on issues like the health implications of vaping and smoking; worsening climate change and how it might sink some of the cities in the country; and cybersecurity and digital privacy amid cases of financial cybercrimes—among many more science-related events and news this year.
READ: Empower communities vs climate change through risk management – experts
READ: Vape Bill: Unresolved debate on vaping’s risks, benefits now to reach Duterte’s table
READ: Threat awareness high as digital banking users list preferred security steps
As the year draws to a close, INQUIRER.net lists some of the biggest local and global science events to look out for in 2023.
Local: Virology and vaccine institute
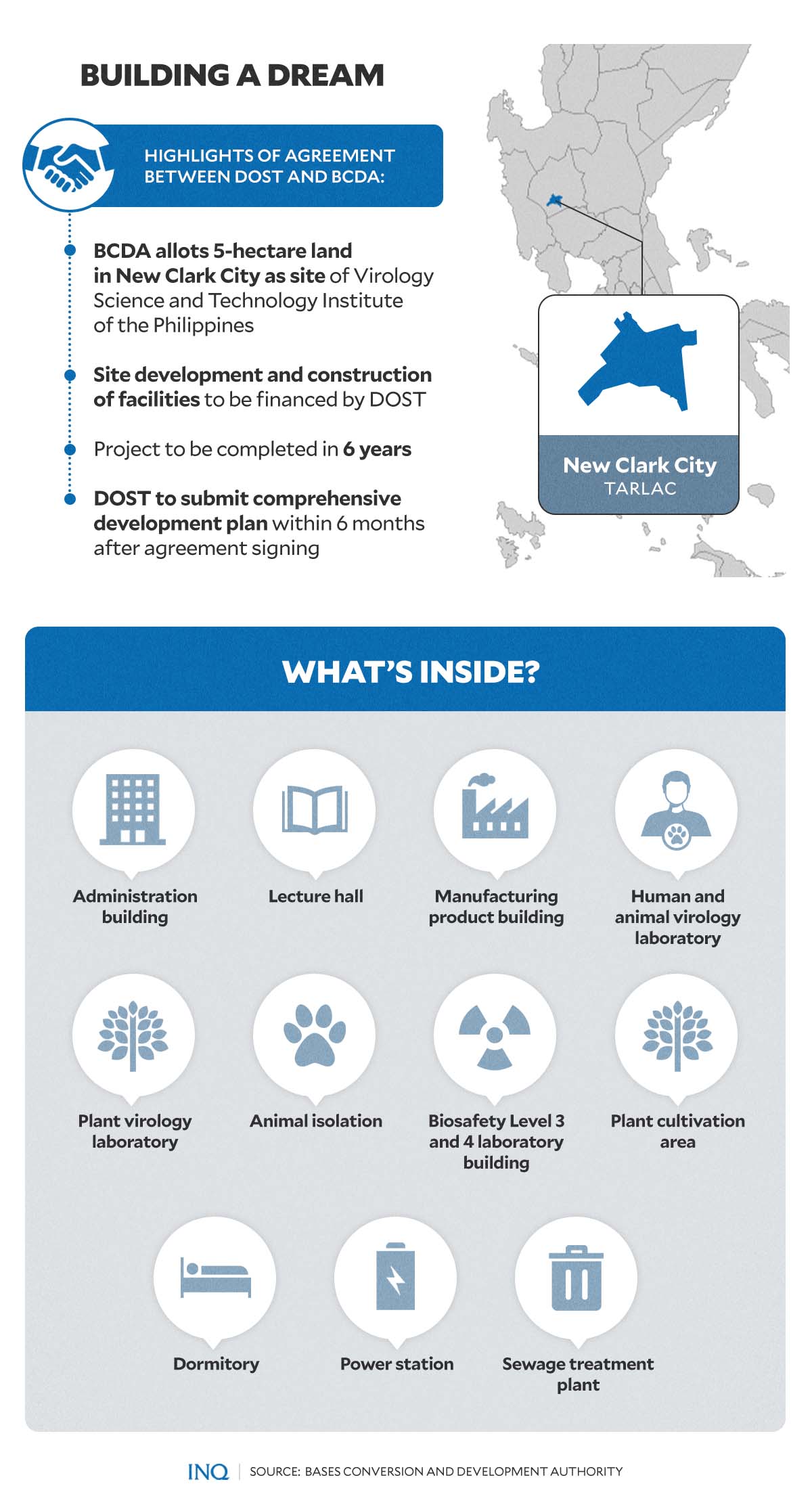
This year, the Bases Conversion and Development Authority (BCDA) announced that it had recently signed an agreement with the Department of Science and Technology (DOST) for the establishment of the country’s “premier virology research institute.”
The proposed Virology and Vaccine Institute of the Philippines (VIP), set to rise on a five-hectare land in New Clark Clark Economic Zone in Capas, Tarlac, will house facilities and laboratories needed for research and development and virology-related projects.
READ: PH virology institute: What to know
GRAPHIC Ed Lustan
Last May, Science and Technology Undersecretary for Research and Development Rowena Cristina Guevara said the construction of the VIP may be done by the end of 2023 or in 2024. While waiting for the construction’s completion, the virology institute can still be partially operated at the DOST’s Industrial Technology Development Institute.
READ: PH’s Virology Institute to rise in end-2023 or in 2024 — DOST
GRAPHIC Ed Lustan
The House of Representatives last December 5 approved on third and final reading the measure pushing for the creation of VIP, also known as House Bill No. 6452 or the Act Establishing the Virology and Vaccine Institute of the Philippines.
READ: Creation of virology, vaccine institute in PH secures House nod on final reading
Local: R&D and virology projects, ‘Balik Scientist’ program
While the construction of the VIP is still ongoing, the DOST announced this year that it had already initiated research and development works for the VIP program.
Among these projects to watch out for in 2023 include six projects in partnership with the Baylor College of Medicine in the United States, St. Luke’s Medical Center, and Research Institute for Tropical Medicine.
“The six projects will focus on the fields of human, animal, and plant virology and the research areas of diagnostics, therapeutics, and vaccines,” said Guevara.
The DOST, as well as President Ferdinand “Bongbong” Marcos Jr., has also urged the participation of Filipino scientists abroad in the VIP program through the “Balik Scientist” program.
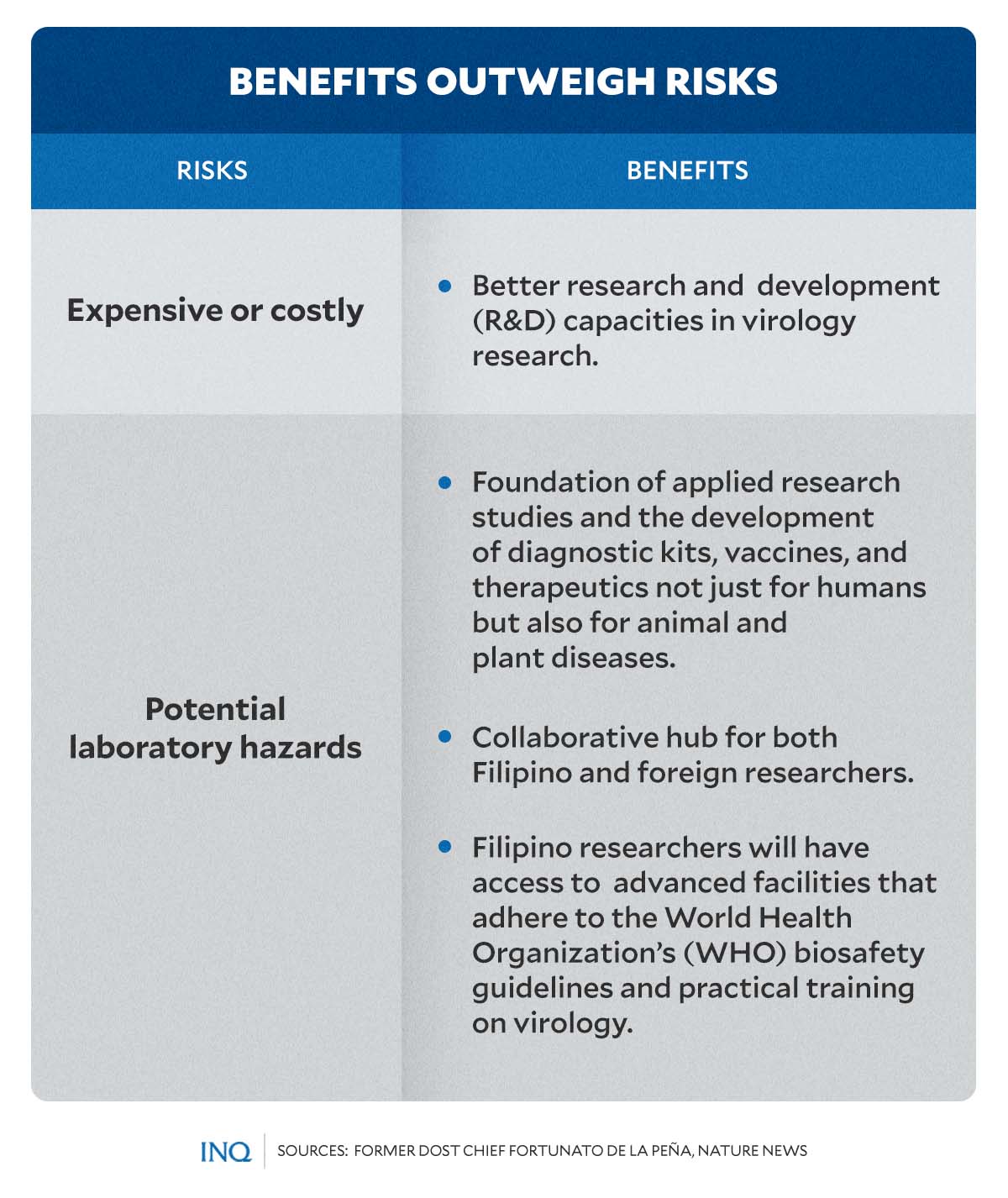
READ: PH virology, vaccine institute: Benefits outweigh risks
READ: Focus on benefits, manage expectations of PH virology institute
Local: Bivalent vaccines in PH
As the country continues to vaccinate its population against COVID in 2023, the government said it is eyeing the procurement of bivalent vaccines early next year.
Bivalent vaccines contain a genetic recipe that can detect and fight both the original strain of the SARS-COV2 virus and Omicron sub-variants BA.4 and BA.5.
According to the US FDA, “[t]he authorized bivalent COVID-19 vaccines, or updated boosters, include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages to provide better protection against COVID-19 caused by the Omicron variant.”
Data from the Department of Health (DOH) showed that as of December 14-15, the cumulative count of sequenced Omicron BA.4 and BA.5 cases in the country had already reached 324 and 12,565, respectively.
So far, the companies currently manufacturing and distributing updated vaccines include Pfizer-BioNTech, Moderna, Sinovac, and the China National Biotec Group—a Sinopharm subsidiary.
READ: What to know about ‘bivalent vaccines’ that target Omicron
Last December 17, the DOH said it aims to procure doses of bivalent vaccines by the first quarter of 2023.
“As for the DOH, we are already coordinating with suppliers for the procurement of bivalent vaccines. We are targeting to have vaccines available by Q1 of 2023. We are also coordinating with potential donors of COVID-19 bivalent vaccines,” the DOH said.
READ: DOH aims to have bivalent vaccines available by Q1 2023
Local: Nuclear power deals, adding nuclear to PH energy mix
Months before the end of his single six-year term, President Rodrigo Duterte signed Executive Order No. 164, which outlined the national government’s position for a nuclear energy program—described as “a process that starts with the inclusion of nuclear power in the energy mix based on a pre-feasibility study on the need for and viability of nuclear power.”
“It includes the development of nuclear power infrastructure and encompasses the planning and construction, operational, commercial, and post-operational stages of nuclear power plants,” the EO stated, adding that it would examine the opening of the mothballed Bataan Nuclear Power Plant (BNPP)..
READ: Adding nuclear to PH energy mix: Easier said than done
During the presidential campaign, Marcos also mentioned the possibility of tapping experts to look into the BNPP and determine if it can still be reopened or if a new one should be constructed despite warnings from experts who believed the move could be risky due to safety issues.
READ: Groups see ‘mixed signals’ in Bongbong Marcos’ renewable energy push
READ: ‘Antiquated’ Bataan nuke plant won’t solve power crisis – scientists
Last November 30, the Philippine Nuclear Research Institute (PNRI) said the Philippine government is looking into possible nuclear deals with South Korea, France, and China as Marcos considers the construction of nuclear power plants to supply the country’s growing energy needs.
READ: PH ramping up talks on nuclear power deals
International: Pathogen watchlist
The World Health Organization (WHO) is expected to publish the revised list of priority pathogens—“agents that can cause outbreaks or pandemics”—in the first quarter of 2023.
The list, according to WHO, will help guide global investment and research and development (R&D)—especially in vaccines, tests, and treatments—in countries across the globe, including the Philippines.
The pathogen watchlist was first published in 2017, while the last prioritization exercise was done in 2018.
Among the agents currently included on the list were COVID-19, Crimean-Cong haemorrhagic fever, Ebola virus disease and Marburg virus disease, Lassa fever, Middle East respiratory syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS), Nipah and henipaviral diseases, Rift Valley fever, Zika and Disease X.
“This list of priority pathogens has become a reference point for the research community on where to focus energies to manage the next threat,” Dr. Soumya Swaminathan, WHO chief scientist, said.
International: The future of mRNA vaccines
The success of messenger RNA (mRNA) technology-based vaccines against COVID pushed vaccine manufacturers and pharmaceutical companies Moderna and Pfizer to develop and test a series of new mRNA vaccines that target other viral diseases.
Earlier this year, both Pfizer and Moderna announced that they will develop its mRNA-based vaccine against shingles—a viral infection caused by the varicella-zoster virus, which leads to painful skin rash and blisters.

GRAPHIC Ed Lustan
BioNTech already began its first-in-human Phase 1 clinical research study for its BNT163 herpes vaccine candidate designed to prevent HSV-2—also known as herpes simplex virus—which causes genital herpes, a sexually transmitted disease.
The German vaccine maker also noted that the BNT163 could also potentially HSV-1, which causes oral herpes and could eventually lead to genital herpes.
TSB
Subscribe to INQUIRER PLUS to get access to The Philippine Daily Inquirer & other 70+ titles, share up to 5 gadgets, listen to the news, download as early as 4am & share articles on social media. Call 896 6000.